Solved 2 NaN3(s) > 2 Na(s) + 3 N2(g) Nitrogen gas has a
In this video we'll balance the equation NaN3 = Na + N2 and provide the correct coefficients for each compound.This is the reaction that takes place when an.
SOLVED Automotive air bags inflate when sodium azide explosively to its constituent
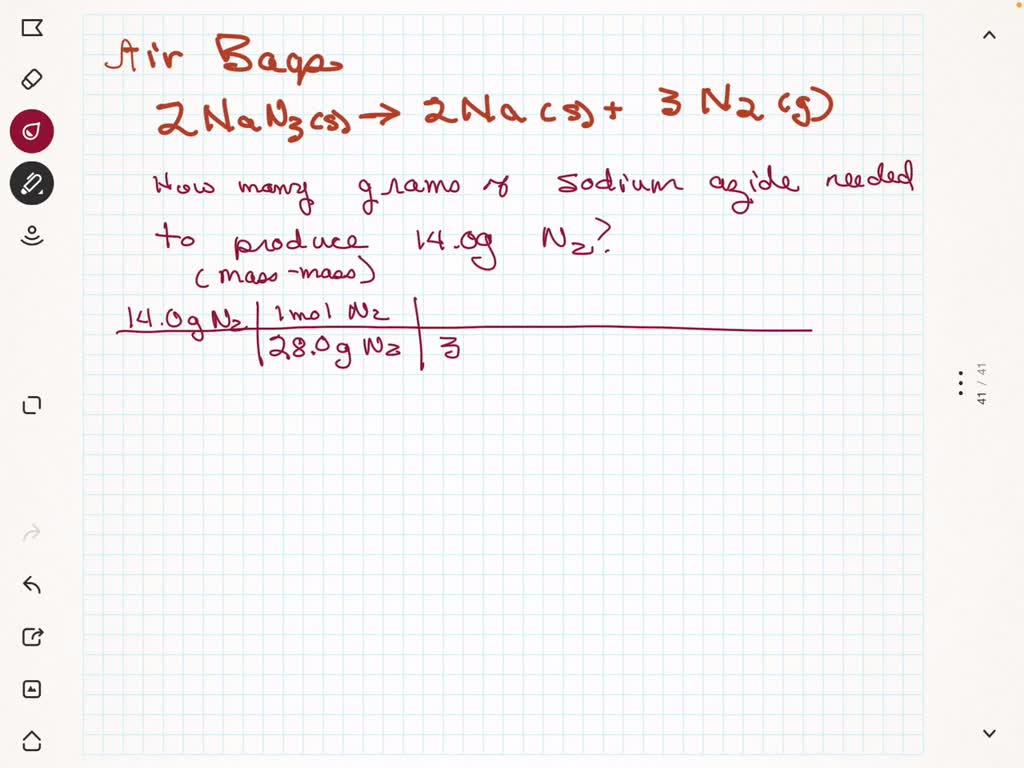
$$\ce{10Na(s) + 2KNO3(s) -> K2O(s) + 5Na2O(s) + N2(g)}$$ a) In what ratio by mass must the sodium azide and potassium nitrate be mixed in order that no metallic sodium remains after the reaction? b) Calculate the total mass of the solid mixture needed to inflate a $60.0\ \mathrm{dm^3}$ air bag at room temperature and atmospheric pressure.
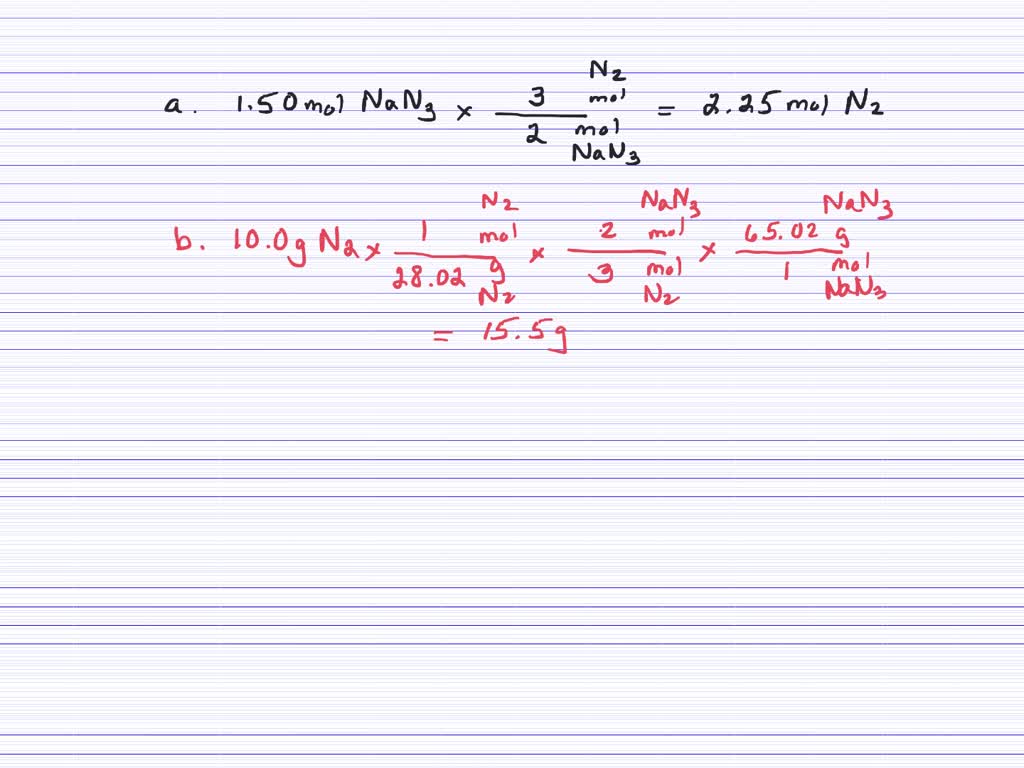
SOLVED What mass in grams of NaN3 is required to produce 50.2 g of N2 gas (density = 1.25 g/L
This includes the Los Angeles Metropolitan Area, Orange County, parts of the High Desert and the Eastern Sierras. A list of other websites in California may be found on the NA Links Page. Regional Helpline Numbers. English: 800-TODAYNA. 800-863-2962. Espanol: 888-NAAHORA. 888-622-4672.
SOLVEDAutomobile air bags inflate following a serious impact. The impact triggers the chemical
NaN3 = Na + N2 - Ecuación química balanceada. Ecuación química balanceada. 2 NaN 3 → 2 Na + 3 N 2. reacción química . Azida De Sodio = Sodio + Dinitrógeno. estructura química. Tipo de reacción. Descomposición. Reactivos. Azida De Sodio - NaN 3. NaN3 Masa molar NaN3 Número de oxidación.
Solved A sample of NaN3(s) fully into Na(s) and

Assign one of the coefficients to 1 and solve the system. a = 1. c = 2 a = 2. d = 6 a / 2 = 4. b = (2 c + d) / 2 = (2 * 2 + 3) / 2 = 3.5. Adjust coefficient to make sure all of them are integers. b = 3.5 so we need to multiple all coefficient by 2 to arrive at the balanced equation with integer coefficients:
SOLVED Q/The of sodium azide (NaN3) has been used to rapidly inflate automobile

Question: 2 NaN3 → 2 Na + 3 N2How many moles of N2 can be created from 3.6 g of NaN3? 2 NaN3 → 2 Na + 3 N2. How many moles of N2 can be created from 3.6 g of NaN3? There's just one step to solve this. Who are the experts? Experts have been vetted by Chegg as specialists in this subject.
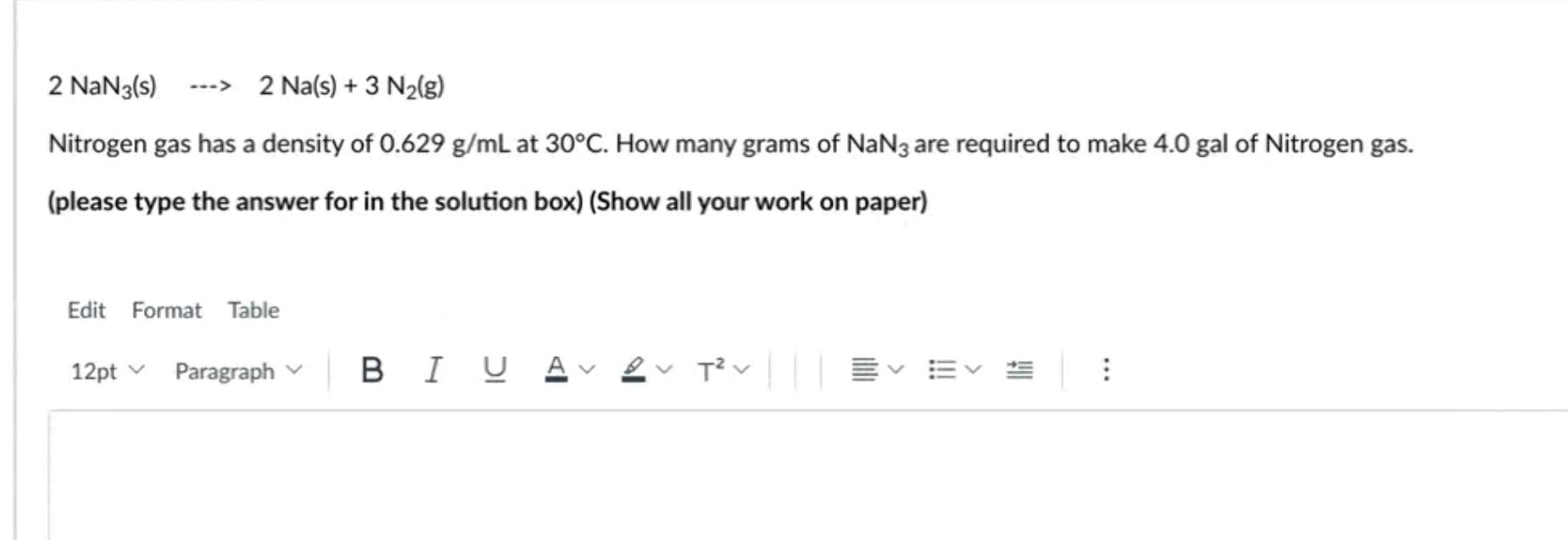
Solved 16. 2 NaN3(s) 2 Na(s) + 3 N2(g) What mass of sodium

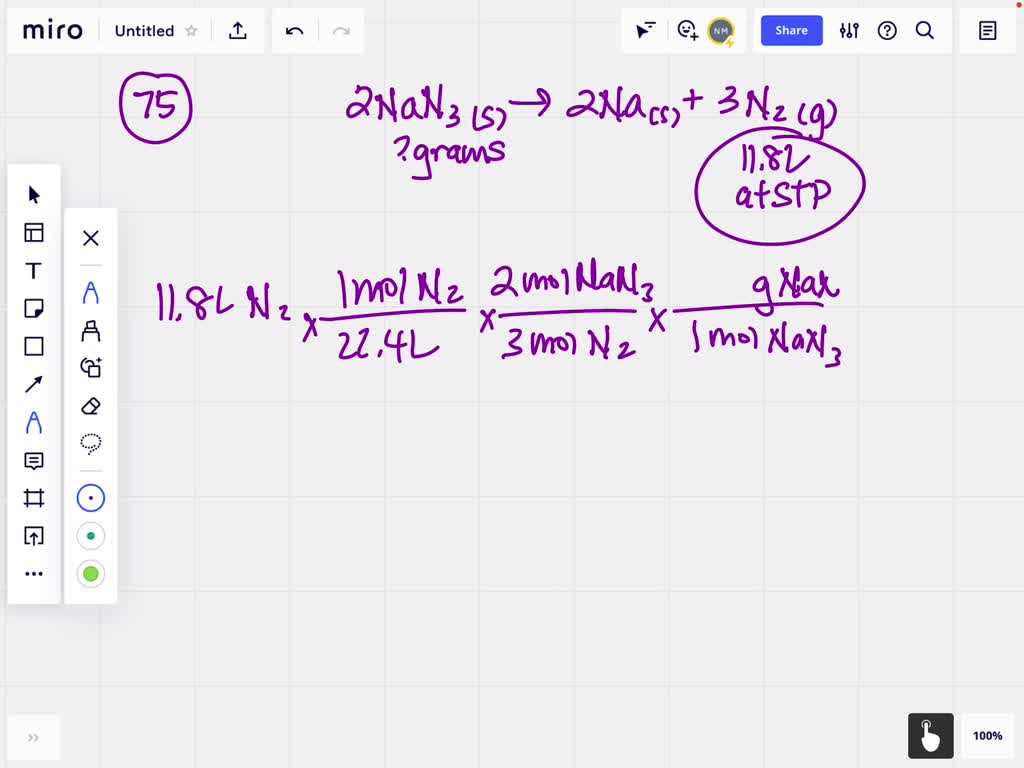
Transition Metals and Coordination Compounds 3h 14m. Automobile air bags inflate following a serious impact. The impact triggers the chemical reaction: 2 NaN3 (s)¡2 Na (s) + 3 N2 ( g) If an automobile air bag has a volume of 11.8 L, what mass of NaN3 (in g) is required to fully inflate the air bag upon impact? Assume STP conditions.
SOLVEDAutumotive air bags inflate when sudium azide, NaN 3, rapidly to its component
Amino acid transmitters provide the majority of excitatory and inhibitory neurotransmission in the nervous system. The sensory-to-motor neuron connection in the spinal cord that controls the knee-jerk reflex is an excellent starting point for illustration. Figure 13.1 shows a monosynaptic connection in the spinal cord between the sensory neuron (in green) and the motor neuron innervating the.
Answered What mass in gram NaN3 is required to… bartleby
Question: Balance each of the following chemical equations. Give your answer as coefficients separated by commas. NaN3(s)→Na(s)+N2(g) Express your answers as integers separated by commas HCl(aq)+O2(g)→H2O(l)+Cl2(g) Express your answers as integers separated by commas.
SOLVED what mass in grams of NaN3 is required to produce 50.2 L of N2 gas (density=1.25 g/L

Norma C. Salceda, MD, F.A.C.O.G. OB-GYN located in Harvard Heights, Los Angeles, CA & Pacoima, CA. Call Us Book Online "Dr. Salceda is an amazing doctor, she explains everything that you need to know." Karin D. Yelp "Dr. Salceda is a wonderful physician. She helped me a lot before and after surgery.
SOLVED The following reaction occurs in an automobile air bag in which sodium azide
The stoichiometric mole ratio of NaN3 for a maximum theoretical yield is 2, which will yield Na and N2 in a ratio of 2:2. If you combine 65.01 g (or 1 moles) of NaN3 (molar mass = 65.01 g/mol) it will have a theoretical maximum yield of 22.99 g (1 moles) of Na (molar mass = 22.99 g/mol) and 42.02 g (1.5 moles) of N2 (molar mass = 28.013 g/mol).
SOLVED When sensors in a car detect a collision, they cause the reaction of sodium acide, NaN3
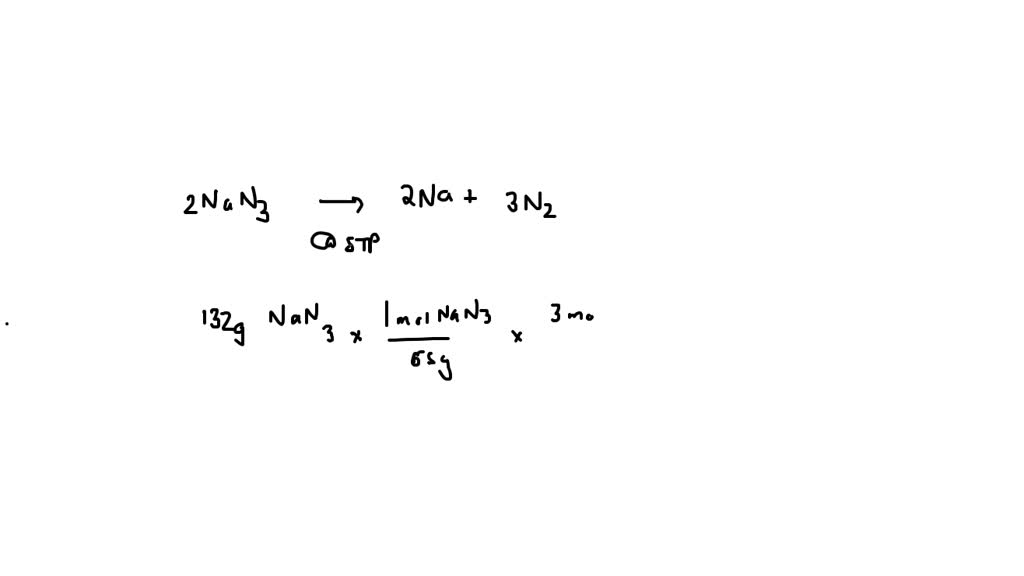
20A-final-practice practice exam balance the equations for the following reactions nan3(s) na(s) n2(g) na(s) fe2o3(s) na2o(s) fe assume that 65.1 of n2 gas are. Skip to document. University; High School. Books; Sign in. Guest user Add your university or school. 0 impact. 0 Uploads. 0 upvotes.
SOLVED An airbag is deployed by utilizing the following reaction (the nitrogen gas produced

Solved 4. Upon heating, NaN3(s) to Na) and N2(g).

_NaN3(s) ----> _Na(s) + _N2(g) 3 Calculate the grams of sodium azide (NaN3) required to produce the number of moles of nitrogen gas calculated in Question 2. 4 How many grams of sodium metal are produced in the decomposition of sodium azide shown in Question 2?
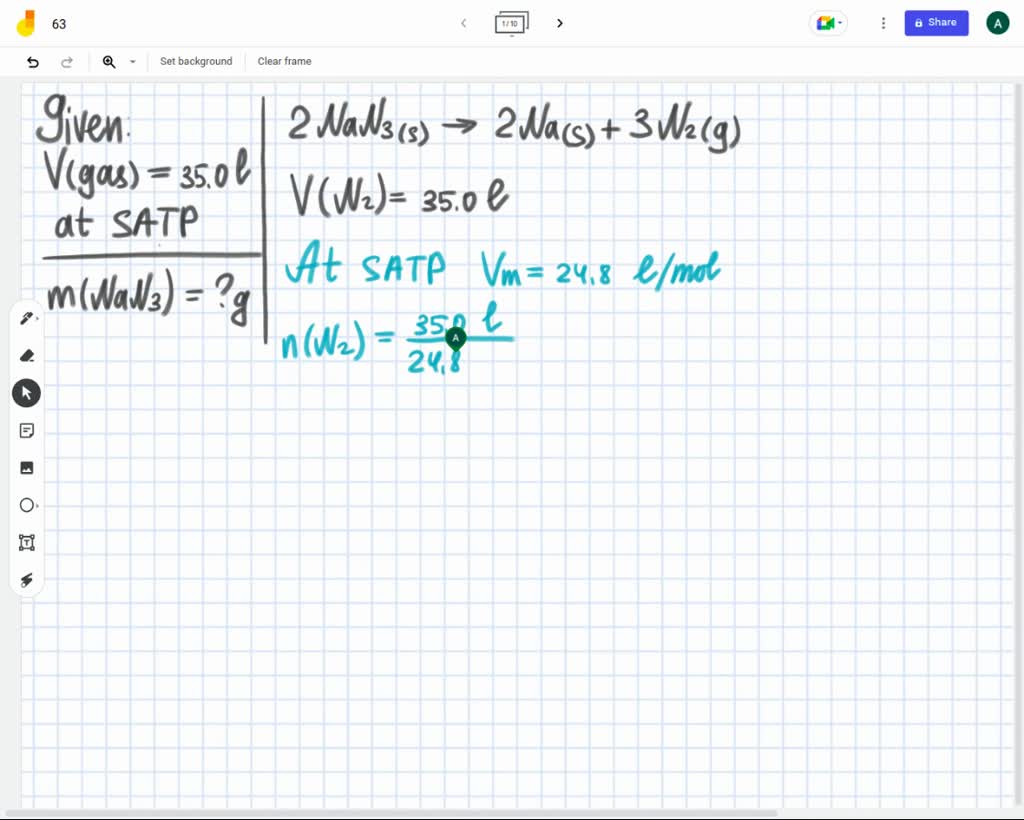
SOLVED 2 NaN3(s) ——> 2 Na(s) + 3 N2(g) How many grams of nitrogen are produced when 10. grams

In order to balance Na on both sides we: Multiply coefficient for Na (s) by 2. 2 NaN 3 (s) = 2 Na (s) + 3 N 2 (g) All atoms are now balanced and the whole equation is fully balanced: 2 NaN 3 (s) = 2 Na (s) + 3 N 2 (g) Balancing step by step using the algebraic method. Let's balance this equation using the algebraic method.
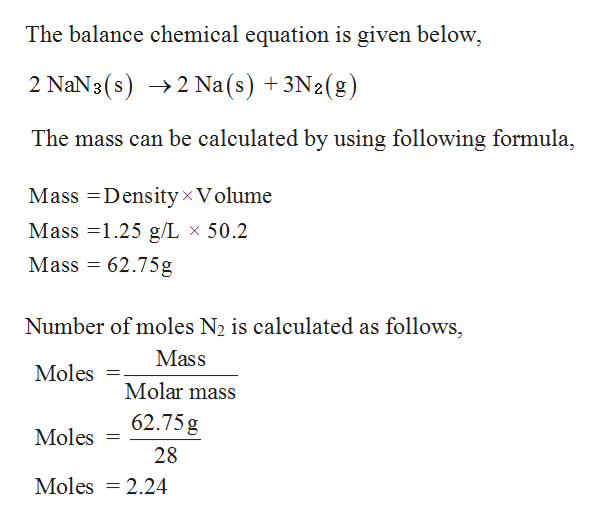
SOLVED Consider the balanced reaction 2 NaN3 (s) → 2 Na (s) + 3 N2 (g) What volume of N2 (in L

NaN3 = Na + N2 is a Decomposition reaction where two moles of aqueous Sodium Azide [NaN 3] decomposes into two moles of solid Sodium [Na] and three moles of Dinitrogen [N 2] gas. Reaction Type. Decomposition. Net Ionic Equation. 2NaN3(aq) = 2Na(s) + 3N2(g) might be an ionic reaction.